Our Passion: support therapy innovations by smart Drug Delivery Devices
We have always been fascinated by the world of combined medicines and the devices required to administer them. Medical devices can deliver much more than just a drug into the body. New technologies help, for example, to achieve more precise dosing, simpler and error-free handling, measurement of therapy adherence and thus help to improve patients’ quality of life.

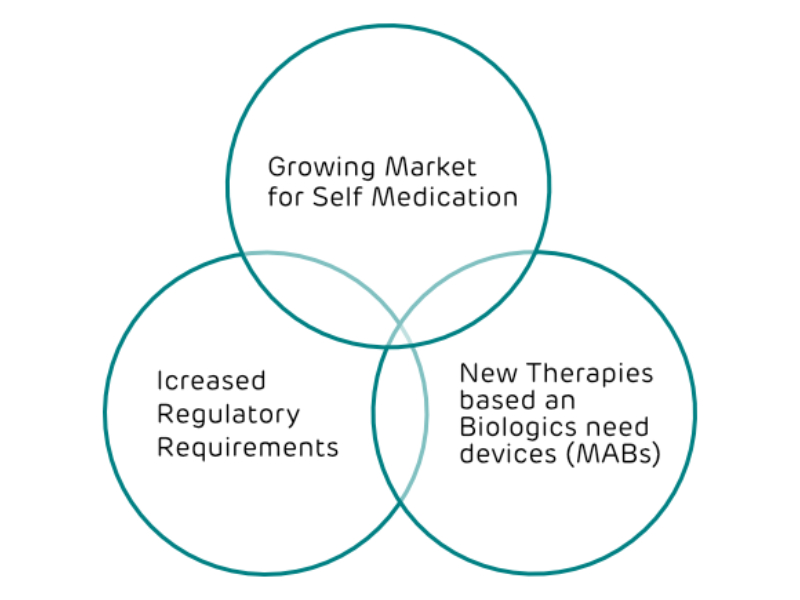
Major Trends in Drug Development
Tomorrow’s Innovative Therapies call for more Drug Device Combinations
More Drug Device Combinations require specialized Technology Providers. Activoris offers a portfolio of technologies for parenteral, pulmonary and ophthalmic delivery devices. Further, we have a proven track record for development and regulatory affairs with drug device combinations.
Learn more about our services in DDC customization projects .
WE ARE LOOKING FORWARD TO YOUR CHALLENGE!
Feel free to send us a personal message or give us a call:
E-Mail: medtech@activoris.com Phone: +49 (0) 6453.585 35 00
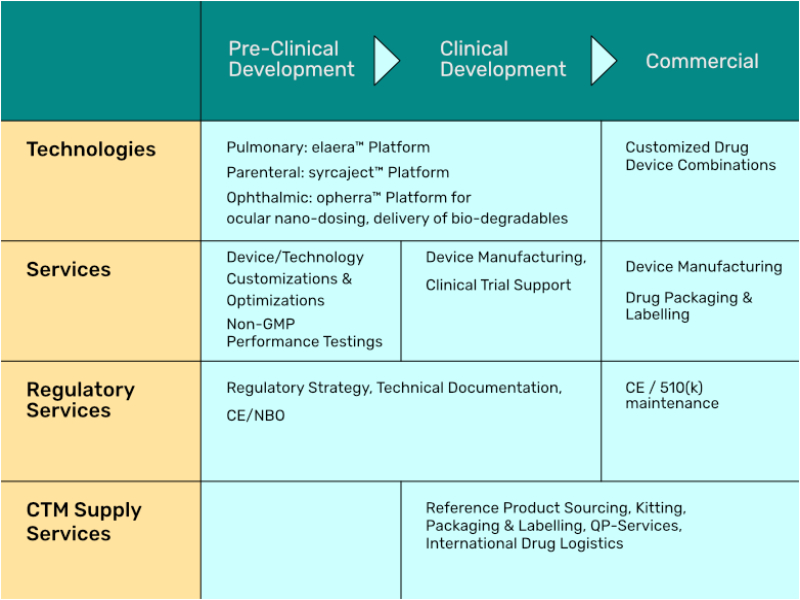
Our Drug Delivery Technologies
Mesh Inhaler Platform for Pulmonary Delivery
With elaera, we can build on decades of expertise in developing premium devices for inhaled drugs. Our development expertise dates back to 1998, when aerosol scientists at the Munich Helmholtz Zentrum invented the ground-breaking nebulizer for controlled breathing, AKITA®.

The new elaera mesh inhaler platform targets challenging compounds, as we see that bio-pharmaceuticals pose higher requirement for particle generation due to fragile molecules and higher viscosities.
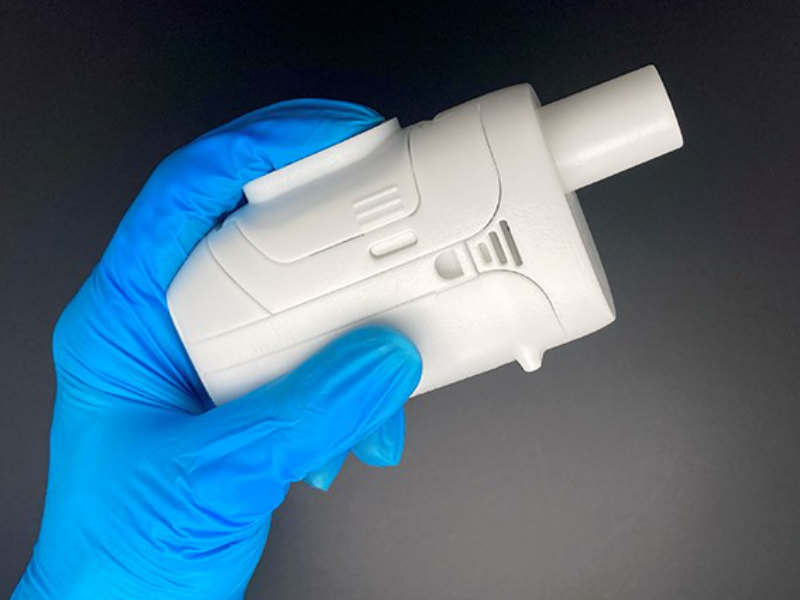
elaera™ state of the art nebulization of challenging bio-pharmaceuticals (prototype)
elaera
- Hand-held mesh nebulizer, breath-triggered, solutions to avoid high velocity inspirations
- State-of-the-Art nebulization performance
- Strong battery, large reservoir
- Exchangeable Dose Control Unit – DCU. Easily adopt the nebulizer performance to specific liquid drug formulation.
- Ready for Connectivity
Drug Dlivery to the Eye
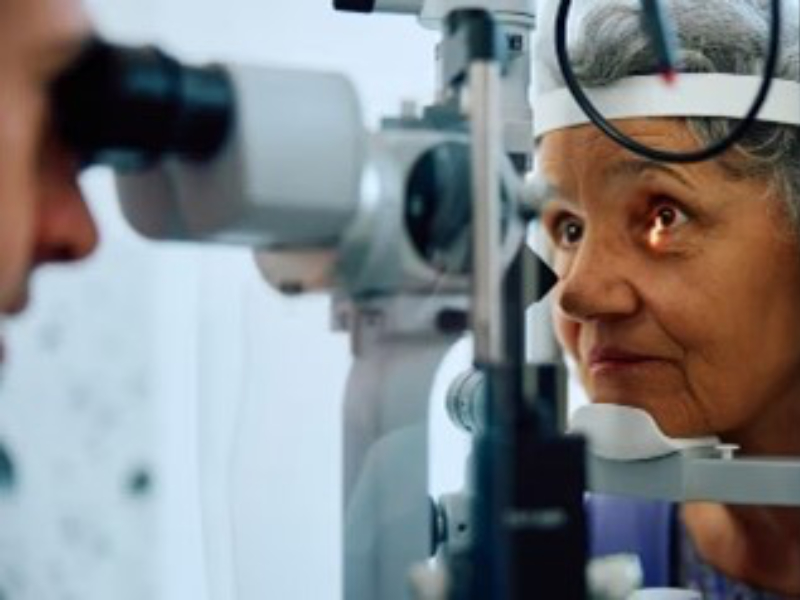
Diseases of the Eye
Age-related macular degeneration (AMD) affects one in eight people 60 years of age or older and is the most common cause of irreversible blindness in older persons in developed countries. According to thorough estimates, 200 million people worldwide are estimated to have AMD, and by 2040, this number is projected to rise to close to 300 million ( Hrishikesh Vyawahare et al, 2022).
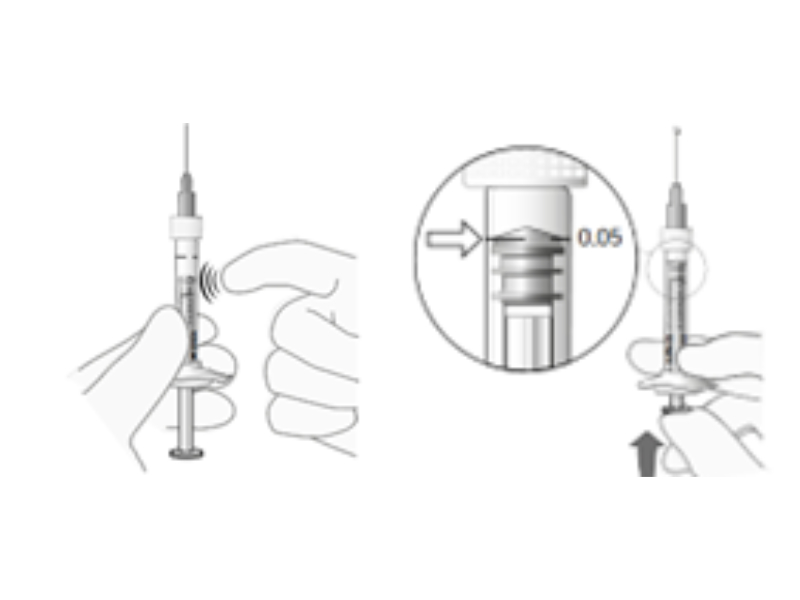
Challenges of delivery of small doses to the Eye
Priming of the syringe is key to avoid injection of air into the eye. A procedure which correctness is still left to the user.
Pharmaceutical developers further move into the direction of applying successful ophthalmic drugs in new indications and patient groups, such as pediatric patients and even pre-mature infants. The doses are down-sized to 20 µl and even less. While not all risks and challenges can be mitigated by a device solution, it is mandatory to further improve safety by reducing use errors.
With opherra™ we are entering the space of ophthalmic delivery devices. Ophthalmic drugs carry the promise of a strong market development, and, at the same time, there is a huge medical need to improve safety and usability of the application to the eye.

We identified several improvements to the current state-of-the art delivery devices, helping to reduce application errors and to provide more comfort the user. Our devices can be combined with different drugs, thus, create a platform to improve many applications. The opherra product group is the perfect life cycle management tool to pro-long your patent protection, thus, safeguarding your market share!
opherra™ liquid µ-dose: standardized priming and dosing
Improved Features for delivery of small µl-Doses:
- Uses a cam box lever to transform travelling way of plunger rod into different propulsions of the plunger
- Defined priming, defined dosing: exact definition of “start of dose” and “end of dose”
- Adaptable to different syringe sizes
- Cam box mechanism enables controlled dosing of high-viscous drugs
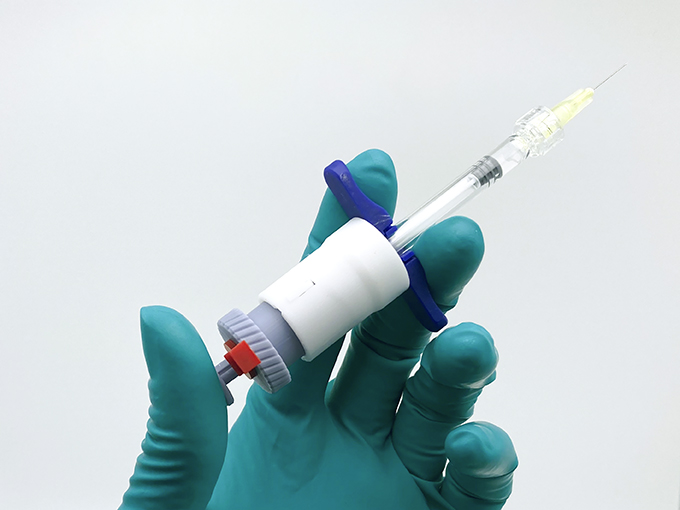
opherra™ microdosing: advanced delivery of potentially high-viscous ophthalmic drugs (prototype)
opherra™ solid: Delivery sustained-release Ocular Implants
Ocular implants present a unique solution to the challenges of treating posterior eye disorders, and many are already commercially available. This delivery route facilitates direct entry of drugs into the blood-retinal barrier and, therefore, highest peak of drug concentration. However, this is the most invasive route of administration, involving penetration of drugs in the eye and, thus, is not free of injection-related complications.
Our opherra solid technology aims to overcome multiple challenges and risk factors of the currently marketed delivery devices for implants.
opherra solid:
- A pen-like hold to aid precision placement.
- Parallel/opposite direction drug release function to avoid added pressure on the eye.
- Reduced speed of injection
- “Priming” pushes drug pellet to the tip of the needle before injection: avoid air bubbles
- Angled plunger design for good finger purchase during release action.
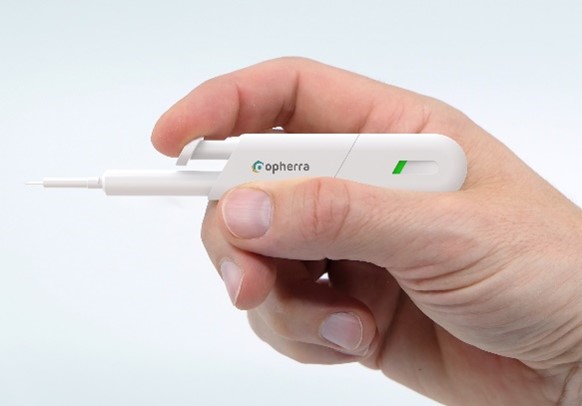
opherra™ solid: advanced delivery of implants (prototype)
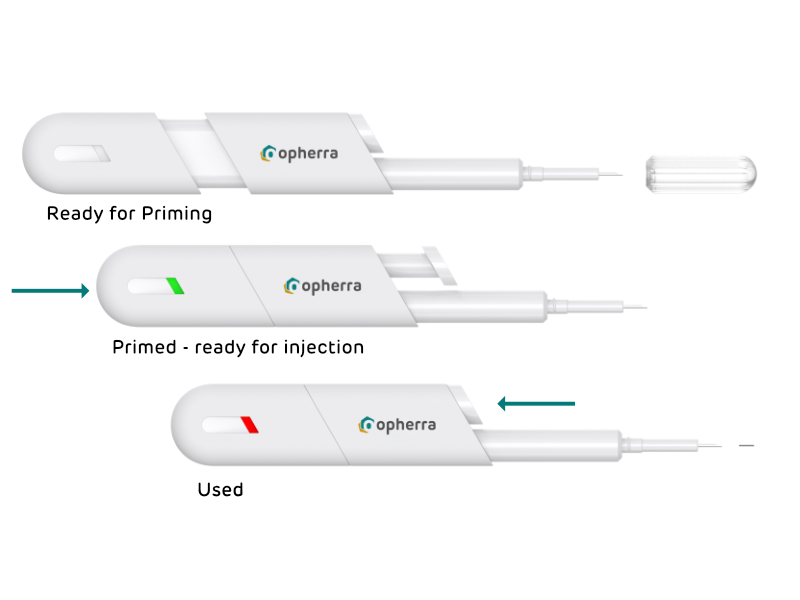
Easy and logic steps for increased safety and comfort of ocular injecitons (prototype).

The indicator in the line of sight (prototype)
Turning a Cartridge into a Pre-Filled Syringe: syrcaject™

Modern drug developments target the market of self-administration with patients suffering from chronic diseases. Prefilled syringes or (auto-)injectors are the devices of choice for the sc/im/iv application of biologics.
syrcaject is our cartridge injector platform, which transforms a simple cartridge into a syringe. Just before use or pre-assembled. syrcaject combines the advantages of the cartridge as a primary container and adds several dosing features.
sysrcaject:
- Combines the advantages of the cartridge as a primary container and adds several features with regards to safe and reprducible dosing. pharmaceutical
- Different needle types possible (luer, staked-in, slip)
- Overcomes challenges of pre-filled syringes
- Drug/device developers can rely on a renown primary container with known properties and with given filling capacities in the industry
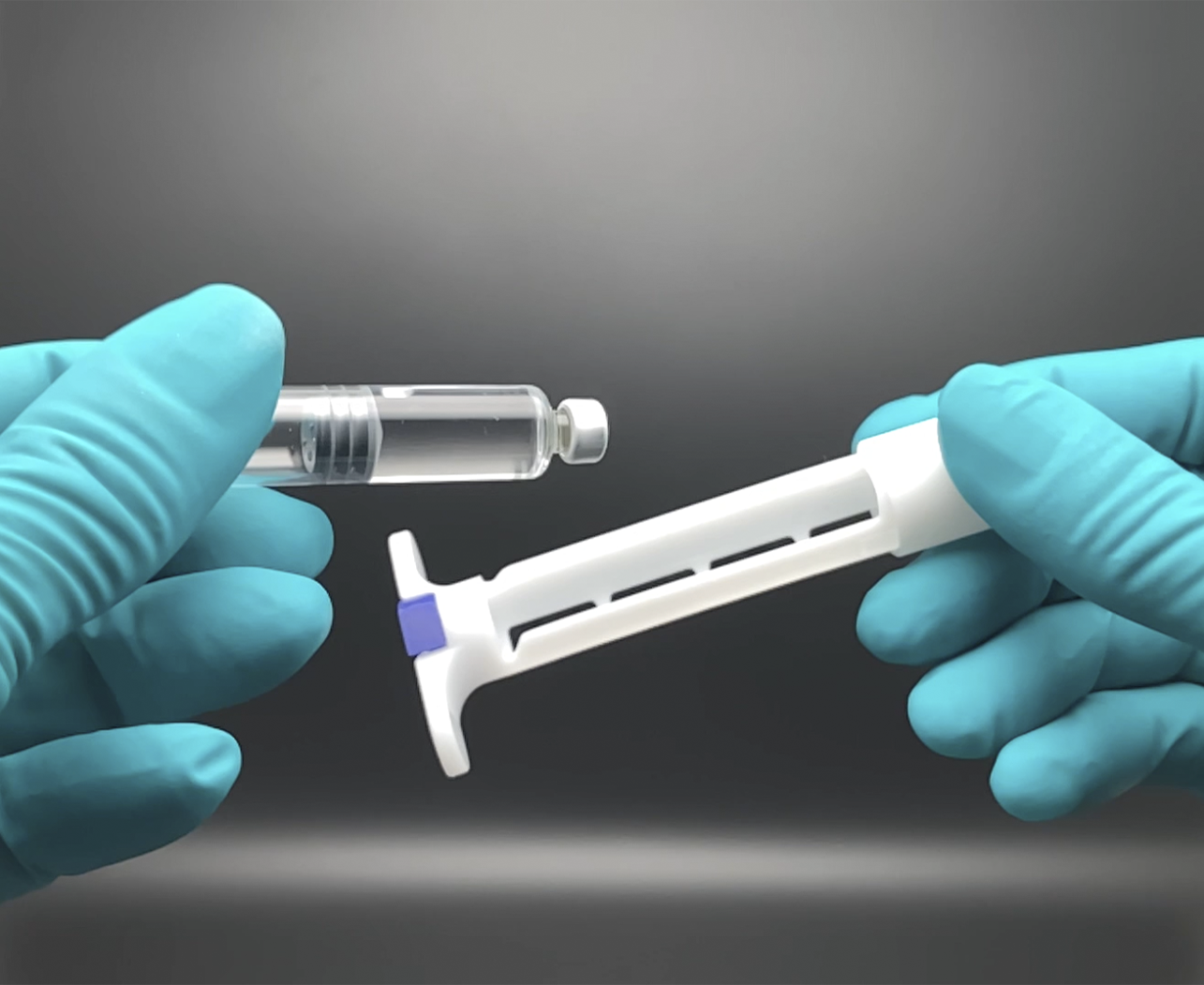
syrcaject™: Turns a cartridge into a syringe (prototype)
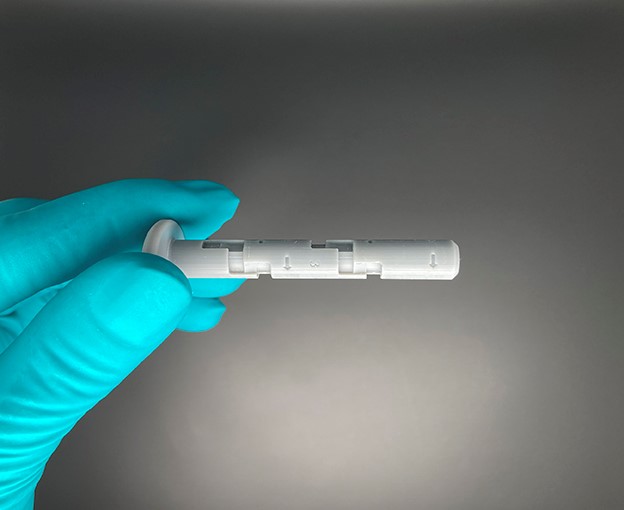

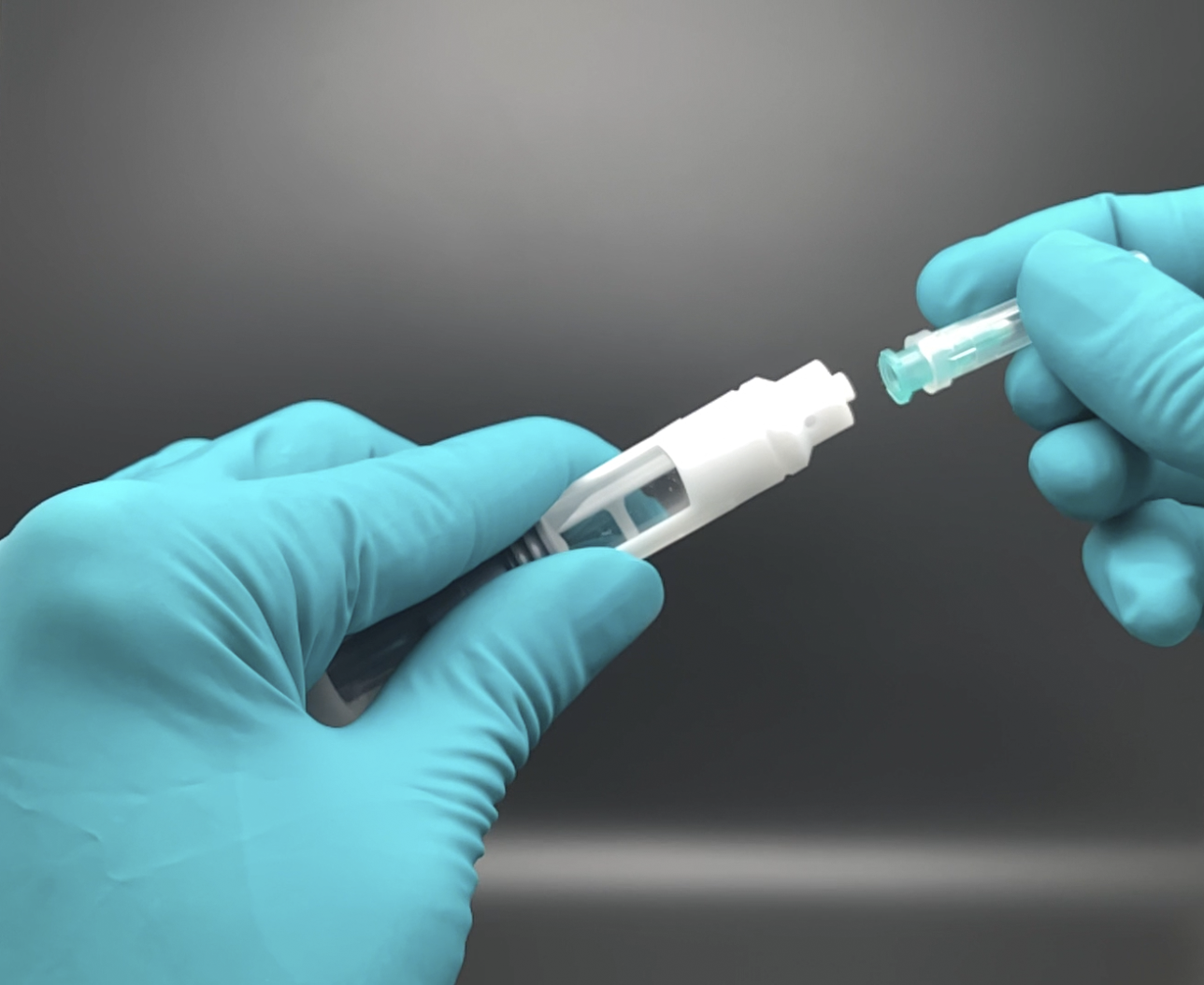
The syrcaject platform comprises the housing for ISO cartridges of different volumes with or without staked needle, or a luer connector. Once the cartridge is inserted a septum needle is in place to penetrate the septum plunger of the cartridge. The shown casing variants can be certified with a CE mark. With the syrcaject technology, biotech companies may realize faster development, better data and reduced development cost. This may be one important argument to perform a clinical study using this technology.
An effective tool for early clinical trials – and more!
Pharmaceutical cartridges are well accepted as primary containers for those applications. Drug development, however, often starts with a vial and transfer syringes, carrying numerous challenges regarding handling and dose accuracy within clinical trials. Further, drug developers have to switch to another primary container (staked-in needle pre-filled syringe or cartridge) later, before phase 3.
Using a cartridge right away allows to speed-up the drug development, bridging of primary containers is no longer necessary. Moreover, flexible and specifically grooved plunger rods allow fixed and reproducible patient doses. Finally, improved safety and efficiency. At the same time: reduced risk.
De-risking and Simplifying the Development Cycle
Early use of cartridges reduces risks and efforts in view of formulation stability programs.
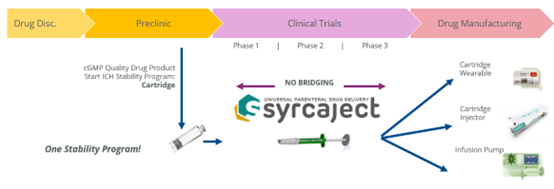
Easy and safe Drug Reconstitution & Transfer

Many drugs for injection need to be reconstituted before use. This involves up to twelve work steps for the user. Work steps that cost time and where mistakes can also be made, meaning compromising patient safety. Our rapimixin system is a simple platform to provide a ready-to-use system to mix a solvent into a drug vial. The platform consists of variants for different primary container carrying a solvent:
- Pharmaceutical Cartridges
- Syringes (luer lock
- BFS amoules
The major advantage is the direct combination of the drug vial, the solvent container with the mixing adapter in one ‘sales unit’. No change of the primary packaging which oftenly is a vial holding the powder or a lyophilized drug. The combination can then be terminally gas-sterilized. Rapimixin then has the opportunity to different interface:
- Needle
- Other drug containers

rapimixin:
- One hand operation possible
- Combines dissolution and transfer in one device
- No change of the primary drug container
- Can come pre-packed and terminally sterilized.
- Takes up to a 5 ml pharmaceutical ISO cartridge.
- Concentration can be pre-determined: reduced source of error.
Our services for your customization project
We are the right partner for your projects along the value chain. From start to finish.
You are looking for integrated know-how for combination products with requirements from medtech and pharmaceutical regulations. Our technology platforms, combined with our development and manufacturing capabilities, provide you with services in all phases of development from a single source.

Activoris Medizintechnik GmbH
Seat, Administration, Devlopment, Production
Wohraer Str. 37
35285 Gemünden (Wohra)
Germany
+49.6453.58535.0
+49.6453.58535.25
Development Team Munich
Robert-Koch-Allee 29
82131 Gauting
Germany
+49.6453.58535.61
+49.6453.58535.25
syrcaject- und opherra-Plattformtechnologien entwickelt in Zusammenarbeit zwischen Activoris und Stephen T. Dunne.
Activoris Food Packaging GmbH
Seat & Administration
Wohraer Str. 37
35285 Gemünden (Wohra)
Germany
+49.6453.58535.0
+49.6453.585350.25
Production
An der Lämmerweide 10
34613 Schwalmstadt
+49.6691.91230.0
+49.6691.91230.25